
by Mr. Maroa Noa | May 19, 2025 | Uncategorized
In the dynamic environment of pharmaceuticals and biotech, change is the one thing you can be sure of — new regulations, product launches, organizational restructuring, and shifting market pressures. But what do you do when a key leader departs, a crucial project demands special expertise, or you simply need extra hands without the long-term commitment?
That’s where Carity Pharma Consultancy can help. Our interim consultancy services give life sciences Companies immediate access to seasoned professionals who can bridge the gap, take control, and deliver — without waiting for a lengthy recruitment process.
Why Interim Consultants?
Interim professionals offer more than just temporary cover — they bring experience, objectivity, and results from day one. Common scenarios include:
- Short-term leadership during restructuring or M&A integration
- Project support on the basis of regulatory submissions, inspections, or product launches
- Specialist skills during start-up, scale-up, or site transfer
Interim consultants deliver business continuity, regulatory compliance, and on-time project delivery without long-term headcount commitment.
Carity’s Interim Consultancy Offering
At Carity Pharma Consultancy, we match you with highly experienced professionals to meet your exact needs — from strategic guidance through hands-on operational support across the pharmaceutical lifecycle.
- Interim Leadership & Executive Roles
Step into stability with experienced interim directors or senior managers in:
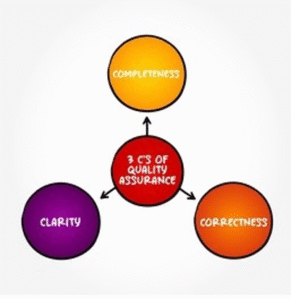
- Quality Assurance – three related categories work together to accomplish quality assurance. To envision the best quality, Carity helps you ensure that both clarity, completeness, and correctness are assembled to serve a common purpose. Clarity refers to the absence of misinformation. Completeness tells that no essential part is missing, whereas correctness elaborately tells that whatever is provided is what is right and has not been modified to suit a certain interest.
- Qualified Person / Responsible Person – The Responsible Person (RP) and Qualified Person (QP) both play crucial roles in the supply of quality and safety products. While the QP is mandated by statute to be responsible for batch release, RP has all accountability in the process of distribution and ensuring that all conforms with good distribution practice (GDP) rules.
- Project-Based Expertise
Get on-demand talent to drive forward high-impact projects:
Clinical development or market access strategy – they attend to different aspects of product lifecycle. Clinical development focus on the entire process of bringing a new drug or device to market, from pre-clinical work to clinical trials and regulatory clearance.
Market access strategy, on the other hand, focuses on ensuring that patients are able to access the drug at a fair price and that it is sufficiently reimbursed.
- Crisis & Turnaround Support
When time is short and stakes are high, we deploy senior consultants who can stabilize operations, resolve non-compliance, and restore regulatory confidence.
- Start-up & Scale-up Assistance
For emerging biotechs and new market entrants, we provide plug-and-play experts to help build foundational systems and processes:
SOP and documentation development – Standard Operating Procedures (SOPs) and documentation are necessary to provide quality, compliance, and process efficiency. SOPs are written, detailed, step-by-step instructions for conducting certain tasks, and documentation is any document that tracks activity, results, and procedures.
GxP compliance frameworks – it is a set of quality requirements and regulations designed to ensure the safety, efficacy and quality of pharmaceutical products. These practices, including Good Manufacturing Practice (GMP), Good Laboratory Practice (GLP), and Good Clinical Practice (GCP), are crucial building public trust and protecting patients in pharmaceutical products.
What makes Carity the best for Interim Support
Industry-Proven Talent: Our network includes former QA heads, regulatory leads, QPs/RPs, and cross-functional experts with deep GxP experience.
Rapid Deployment: We understand time is critical — our consultants can begin work right away, in most instances within days.
Flexible Engagements: From part-time support to full-time leadership, we engage in a manner that fits your requirements and budget.
Seamless Integration: Carity consultants become an extension of your organization, with complete alignment to your culture, processes, and goals.
Trusted Support, When You Need It Most
Whether you’re filling a gap, managing change, or driving growth, Carity Pharma Consultancy provides trusted interim professionals who bring stability, speed, and strategic value. With us, you’re not just filling a seat — you’re gaining a capable partner committed to your success.

by Mr. Maroa Noa | May 19, 2025 | Uncategorized
In Pharma, patient safety doesn’t stop at manufacturing — it extends all the way to product delivery. Ensuring medicines are always handled, transported, and stored under the right conditions isn’t just best practice — it’s also a regulatory requirement. At Carity Pharma Consultancy, our GDP services are designed to help your organization remain compliant, protect product integrity, and build trust along your supply chain.
Why GDP is Necessary
GDP ensures that medicinal products are distributed in a controlled and traceable manner, their quality is maintained, and they are protected against contamination, falsification, or improper handling.
Regulatory agencies and national authorities strictly require compliance with GDP for wholesale distribution authorization, including:
- Compliant and safe transportation – staying in accordance with established specifications and guidelines alongside putting measures that ensure quality, and standard of products during transportation is very significant. This helps them serve the intended purpose without side effects.
- Controlled temperature storage – having a controlled temperature storage ensures that the drugs’ efficacy and integrity is attended by retaining specific temperature ranges.
- Full traceability and documentation – it is crucial for patient safety, quality control and regulatory compliance. Documentation includes regulatory submissions and manufacturing processes’ records.
- Qualification of suppliers and customers – customer and supplier qualification focus on verifying that both customers and suppliers are authorized to handle or receive medicinal products, often through checking licenses and authorization.
- Robust quality systems – this encompasses a structured quality managing technique, it includes, continuous improvement, risk management, compliance with set standards and process monitoring.
Non-compliance can lead to regulatory action, reputational damage, or even worse — patient harm.
Our GDP Consultancy Services
Carity Pharma Consultancy offers end-to-end GDP support depending on your business model — whether you’re a distributor, wholesaler, or logistic provider.
- Application Support
We offer support with preparing, submitting, and chasing Wholesale Distribution Authorisation applications, including:
Development and review of quality systems documentation – organized documents and records that describe the procedures, processes, Outcomes related to product quality and standards.
GDP inspection preparation – risk of non-compliance is minimized during a GDP inspection ensuring efficacy and safety of medical products throughout the distribution channel.
Liaison with competent authorities – laws and regulations enforced by Authorities results to healthy and positive competition.
- Quality Management System (QMS) Development
A strong QMS is the cornerstone of GDP compliance. We help develop and implement:
Standard Operating Procedures – the standard operating procedures are crucial in promoting quality, efficiency and consistency in that, they provide elaborate instructions for tasks ss
Risk management plans and deviation control – this is beneficial in proactively identifying and mitigating potential problems before they effect production. Moreover, deviation control focus on addressing challenges that occur while manufacturing is ongoing.
Quality and compliance audits – patient’s health is ultimately safeguarded through the quality compliance audits by checking product quality.
- Responsible Person (RP) Services
Legislation requires a named Responsible Person to be responsible for GDP compliance. We offer:
- External RP services
- RP training and mentoring for internal candidates
- Responsible Person oversight support for multiple sites or regions
- GDP Audits & Gap Assessments
We uncover gaps and help you strengthen your compliance status, some of the most significant gaps we uncover include;
Internal audits of GDP processes – the entire supply chain’s integrity, compliance and quality which is significant hugely rely on Good Distribution Product’s internal audit.
Supplier and transport partner audits – to maintain public trust and safeguard pharmaceutical products’ integrity, it is significant to do transport partner and supplier audits.
Corrective and preventive action (CAPA) planning – some poor habits need to be dropped to succeed, likewise, it is good to correct and improve when necessary, to tell areas that need to be improved, it is advisable to do audits and adjust on the shortcomings.
- Training & Continuous Improvement
We train your staff to maintain and improve GDP standards:
Tailored GDP training sessions – to always improve and maintain GDP standards, training your team is very essential. Our team that is specialized on GDP standards matters is very reliable and should be your destination.
Onboarding programs for logistics staff – for efficient service delivery, having an organized staff loaded with significant programs is very ideal, we offer that service as well.
Periodic refresher courses and compliance updates – market dynamics keep shifting, the periodic refresher courses and compliance updates keeps you to the latest market
Why prioritize Carity?
Deep Regulatory Knowledge: Our consultants have hands-on experience with MHRA and EU GDP regulations.
Flexible Support: End-to-end support or specific interventions – we adapt to your needs.
Client Trusted: Start-ups to global distributors rely on Carity for solid, pragmatic GDP guidance.
Strengthen Your Supply Chain, Ready?
Pharmaceutical distribution is under the spotlight more than ever, and compliance isn’t optional. With Carity Pharma Consultancy on your side, you can trust a distribution system that’s compliant, secure, and inspection-ready.
We are open to discuss and outline how our GDP consultancy services can support your business goals and regulatory needs, without limiting your engagements with our specialized team.
by Mr. Maroa Noa | Mar 28, 2025 | Uncategorized
Access to essential medicines remains challenging in many regions, including Kenya, where certain prescription and orphan drugs are either unavailable or difficult to source. Often crucial for treating rare diseases, these medications require specialized procurement strategies to ensure they reach the patients who need them most. At Clarity Pharma Consultancy, we provide expert pharmaceutical sourcing services, bridging the gap between global pharmaceutical suppliers and the Kenyan healthcare sector.
Understanding Orphan Drugs and Their Importance
Orphan drugs are specialized medications developed to treat rare diseases that affect a small percentage of the population. Due to the limited number of patients requiring these treatments, pharmaceutical companies often face challenges in making them widely available. Consequently, regulatory hurdles, high costs, and logistical difficulties contribute to the scarcity of these drugs in Kenya. However, ensuring access to orphan drugs is vital for improving the quality of life for patients with rare conditions.
Challenges in Accessing Prescription Drugs in Kenya
Several factors contribute to the unavailability of specific prescription and orphan drugs in the Kenyan market:
- Regulatory Barriers: Kenya’s pharmaceutical regulatory framework ensures drug safety and efficacy but can sometimes slow down the approval process for specialized medications.
- Limited Market Demand: With a smaller patient base, pharmaceutical companies may not prioritize distributing orphan drugs to Kenya, making them difficult to obtain.
- High Costs and Supply Chain Limitations: Importing rare drugs requires significant investment in logistics, proper storage conditions, and regulatory approvals, increasing costs for both suppliers and patients.
- Lack of Awareness: Many healthcare providers and patients may not be aware of alternative sourcing options for unavailable drugs.
How Clarity Pharma Consultancy Ensures Access to Essential Medications
At Clarity Pharma Consultancy, we specialize in the sourcing of orphan drugs and unavailable prescription medications, helping healthcare providers and patients access life-saving treatments. Our pharmaceutical consulting services include:
- Market Analysis and Feasibility Assessment: We evaluate market potential, production volumes, and demand for specific drugs to create viable sourcing strategies.
- Global Supplier Network: Through our extensive international partnerships, we identify and collaborate with manufacturers and authorized distributors to procure high-quality, certified medications.
- Regulatory and Compliance Support: Navigating Kenya’s pharmaceutical regulations can be complex. We offer expert guidance in securing the necessary approvals for importation and distribution.
- Logistics and Distribution Management: We ensure safe, efficient transportation, storage, and delivery of temperature-sensitive and high-value medications.
- Customized Solutions for Healthcare Providers and Patients: We tailor our pharmaceutical sourcing services to meet the unique needs of hospitals, clinics, and individuals seeking access to rare medications
Sustainable Solutions for Better Healthcare Access in Kenya
Sourcing orphan and unavailable prescription drugs is challenging, but with strategic planning and expert pharmaceutical consultancy, we provide sustainable solutions to improve healthcare access in Kenya. By leveraging our strong industry connections and expertise, we help patients receive critical treatments that would otherwise be out of reach.
If you are a healthcare provider, patient, or stakeholder looking for assistance in sourcing orphan drugs or unavailable prescription medications, Clarity Pharma Consultancy is your trusted partner. Visit our website to learn more about our pharmaceutical sourcing solutions and how we can support your medical needs.
FAQs
1. Are there legal restrictions on importing orphan and unavailable prescription drugs into Kenya?
Yes, Kenya has strict regulations on importing medications. The Pharmacy and Poisons Board (PPB) oversees the importation of drugs, ensuring they meet safety and quality standards. Special permits may be required for certain medications.
2. How long does it take to source and import these drugs?
The timeline varies depending on the drug, supplier, and regulatory requirements. Some medications can take a few weeks to import, while others requiring special approvals may take longer. It’s best to start the process as early as possible.
3. What should I consider when sourcing these drugs to ensure safety and authenticity?
Always work with reputable suppliers, verify regulatory approvals, check for proper storage and handling, and ensure the drug has valid documentation, including batch numbers and expiry dates. Consulting a licensed pharmacist can help prevent counterfeit or unsafe medication use.

by Mr. Maroa Noa | Mar 27, 2025 | Uncategorized
At Clarity Pharma Consultancy, we get it, navigating the importation process in the pharmaceutical world can be overwhelming. With all the regulations and paperwork involved, it’s no small feat to get your products into the Kenyan market. That’s where we come in. Our goal is to make the entire process smooth, efficient, and fully compliant, so you can focus on what you do best. Let’s break down our importation services, our dedication to excellence, and what makes us stand out in the industry.
Our Importation Services: What We Offer
1. IDF and Permit Applications
Dealing with regulations is the key to successful importation, and we’re here to help you navigate it all:
- IDF Preparation: The Import Declaration Form (IDF) is a must for any pharmaceutical imports into Kenya. We’ll guide you through the process, ensuring all the necessary details like product classification and intended use are spot on.
- Permit Acquisition: We handle the heavy lifting when it comes to getting permits from the Pharmacy and Poisons Board (PPB) and other regulatory bodies. From health and safety standards to final approval, we make sure your products are good to go.
2. Documentation Management
The right documentation can make or break the import process. That’s why we’ve got you covered with:
- Comprehensive Document Preparation: From Certificates of Conformity (CoC) from KEBS to commercial invoices, packing lists, and bills of lading, we ensure everything is in order.
- Regulatory Compliance Checks: We thoroughly review all documents to ensure they meet Kenyan regulations. This minimizes any risk of delays or rejections.
3. Customs Clearance Services
Customs clearance doesn’t have to be a headache. With us, it’s a smooth ride:
- Efficient Customs Processing: Our experienced clearing agents know the Integrated Customs Management System (ICMIS) inside and out, ensuring quick electronic submissions and approvals.
- Problem Resolution: If any issues pop up during customs clearance, we’re on it. Our team works fast to resolve problems and keep your goods moving.
4. Logistics Coordination
Once your products are cleared, we’ll make sure they get to your warehouse without a hitch:
- Transportation Solutions: We partner with trusted logistics providers to deliver your goods safely and on time, with options tailored to your needs.
- Delivery Tracking: Stay in the loop with regular updates on your shipment’s status, so you can plan accordingly.
Why Compliance Is Key
In the pharmaceutical industry, compliance isn’t optional, it’s essential. Kenya has strict regulations to ensure public health and safety, and we prioritize compliance every step of the way.
- Staying Current on Regulations: Our team keeps up with the latest changes in Kenyan laws and requirements for pharmaceutical imports, so you always get accurate guidance.
- Avoiding Risks: By ensuring full compliance, we help you steer clear of fines, delays, or worse product recalls. Protecting your investment and reputation is our top priority.
What Sets Clarity Pharma Consultancy Apart
Expertise You Can Count On
With many years in the pharmaceutical industry, we’ve seen it all. Our team is equipped to handle even the most complex importation challenges.
A Client-First Approach
At Clarity Pharma Consultancy, we believe that placing our clients at the heart of everything we do is essential for achieving mutual success. Our client-centric approach is not just a strategy; it is a fundamental philosophy that shapes our operations, decisions, and interactions.
Competitive Pricing
We offer cost-effective solutions without compromising on quality. By optimizing the importation process, we help reduce unnecessary expenses, ensuring better value for your business.
Fast Turnaround Time
Delays in the import process can cost your business valuable time and money. Our streamlined approach guarantees quicker approvals, faster clearance, and timely delivery of goods.
Commitment to Quality
From meticulous documentation to efficient logistics, we’re all about delivering the highest standards of service. Your products are in safe hands with us.
At Clarity Pharma Consultancy, we’re more than just a service provider, we’re your partner in navigating the complexities of pharmaceutical importation. Let us handle the hard stuff so you can focus on growing your business.




