Who we are & What we can do for you
Our comprehensive import and export solutions are tailored to simplify and streamline these intricate processes, ensuring compliance with all relevant regulations and efficient logistics operations, while minimizing delays and maximizing cost-effectiveness on your behalf. Read More...

How to Maintain GDP Compliance & Excellence
At Carity Pharma Consultancy, our GDP services are designed to help your organization remain compliant, protect product integrity, and build trust along your supply chain. Read More...

Offering Proactive Compliance Assurance through Audits and Mock Inspections
Our audits and mock inspections provide a comprehensive approach to identifying and addressing potential gaps, ensuring your organisation always remains prepared for regulatory inspections. Read More...
Our Team, In the Press
Read our latest updates, in our Blogs

The Kenya’s Legal Intellectual Property Framework
With the increasing recognition of intangible assets, such as patents, trademarks, and industrial designs, in economic development, Kenya has developed and adopted a comprehensive legal framework to govern these rights.

How to Boost Commercial Value
Intellectual property (IP) is not just about safeguarding ideas; it’s also about transforming them into valuable, market-ready assets. Once a patent is granted or a trademark is registered, the next critical step in the innovation journey is commercialisation.
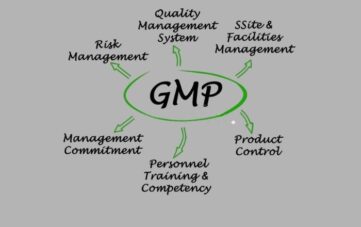
Expert GMP Services for Manufacturing Excellence
Whether you’re establishing new premises, preparing for an inspection, or returning to production following findings of non-compliance, our GMP consultants are behind you to assist in navigating the complexity of pharmaceutical production with certainty and precision.

